what causes oxygen to be released in the tissues? explain your answer.
What is the oxygen dissociation curve?
The oxygen dissociation curve is a graph that plots the proportion of haemoglobin in its oxygen-laden saturated grade on the vertical axis against the fractional pressure of oxygen on the horizontal centrality. The curve is a valuable help in understanding how the blood carries and releases oxygen and it is a common theme that is tested on in many medical examinations.
At high fractional pressures of oxygen, haemoglobin binds to oxygen to class oxyhaemoglobin. All of the blood-red blood cells are in the form of oxyhaemoglobin when the blood is fully saturated with oxygen. Each gram of haemoglobin can combine with ane.34 mL of oxygen. At low partial pressures of oxygen (eastward.thousand. within tissues that are deprived of oxygen), oxyhaemoglobin releases the oxygen to course haemoglobin.
The oxygen dissociation curve has a sigmoid shape because of the branch bounden of oxygen to the four polypeptide chains. Co-operative bounden means that haemoglobin has a greater ability to bind oxygen after a subunit has already bound oxygen. Haemoglobin is, therefore, most attracted to oxygen when three of the 4 polypeptide chains are jump to oxygen.
At that place is often a P50 value expressed on the curve, which is the value that tells us the fractional pressure of oxygen at which the red claret cells are 50% saturated with oxygen. At an oxygen saturation of fifty%, the PaO2 is approximately 25 mmHg (3.5k Pa).
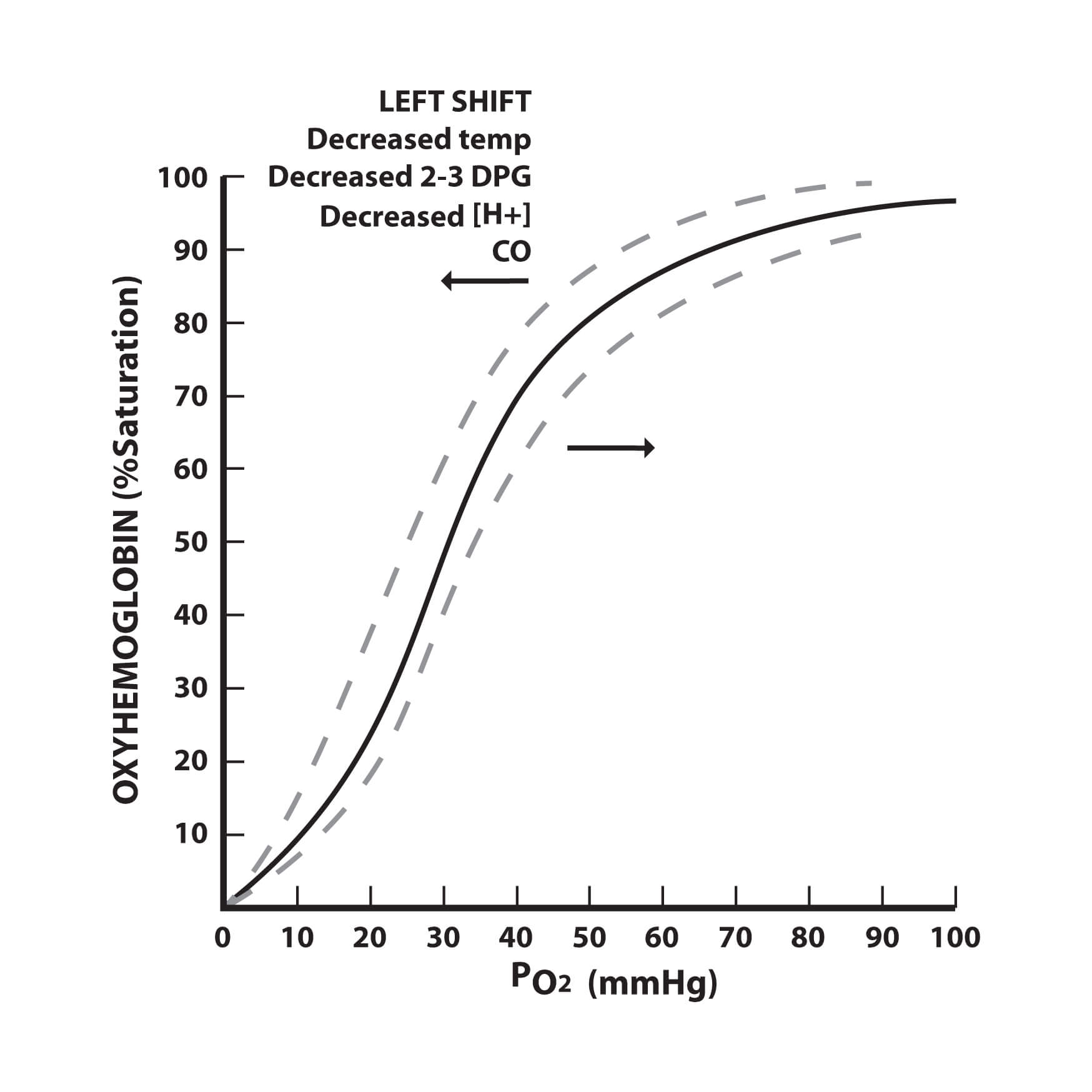
The oxygen dissociation curve and the factors affecting information technology.
Which factors impact the oxygen dissociation bend?
The oxygen dissociation bend can be shifted right or left past a variety of factors. A correct shift indicates decreased oxygen affinity of haemoglobin allowing more oxygen to be available to the tissues. A left shift indicates increased oxygen affinity of haemoglobin allowing less oxygen to be available to the tissues.
pH:
A decrease in the pH shifts the curve to the right, while an increase in pH shifts the bend to the left. This occurs because a higher hydrogen ion concentration causes an alteration in amino acid residues that stabilises deoxyhaemoglobin in a state (the T state) that has a lower analogousness for oxygen. This rightward shift is referred to equally the Bohr result.
Carbon dioxide (CO two ):
A decrease in CO2 shifts the curve to the left, while an increase in CO2 shifts the curve to the right. CO2 affects the bend in 2 ways. Firstly, the accumulation of CO2 causes carbamino compounds to be generated, which bind to oxygen and class carbaminohaemoglobin. Carbaminohaemoglobin stabilizes deoxyhaemoglobin in the T land. Secondly, the accumulation of CO2 causes an increment in H+ ion concentrations and a decrease in the pH, which will shift the bend to the right equally explained to a higher place.
Temperature:
An increase in temperature shifts the curve to the right, whilst a decrease in temperature shifts the bend to the left. Increasing the temperature denatures the bond between oxygen and haemoglobin, which increases the amount of oxygen and haemoglobin and decreases the concentration of oxyhaemoglobin. Temperature does not have a dramatic effect but the effects are noticeable in cases of hypothermia and hyperthermia.
Organic phosphates:
ii,3-Diphosphoglycerate (2,3-DPG) is the principal chief organic phosphate. An increment in 2,iii-DPG shifts the curve to the right, whilst a decrease in 2,3-DPG shifts the curve to the left. 2,3-DPG binds to haemoglobin and rearranges it into the T state, which decreases its analogousness for oxygen.
A table summarizing these furnishings is shown below:
| Cistron | Decrease | Increase |
|---|---|---|
| pH | Right shift | Left shift |
| CO2 | Left shift | Correct shift |
| Temperature | Left shift | Right shift |
| two,three-DPG | Left shift | Right shift |
How does carbon monoxide affect the bend?
Carbon monoxide (CO) interferes with the oxygen transport function of the blood by combining with haemoglobin to course carboxyhaemoglobin (COHb). CO has approximately 240 times the analogousness for haemoglobin than oxygen does and for that reason, even small-scale amounts of CO tin tie up a large proportion of the haemoglobin in the claret making it unavailable for oxygen carriage. If this happens the PO2 of the blood and haemoglobin concentration will be normal just the oxygen concentration volition be grossly reduced. The presence of COHb besides causes the oxygen dissociation curve to be shifted to the left, interfering with the unloading of oxygen.
How does methaemoglobin bear on the bend?
Methaemoglobin is an abnormal form of haemoglobin in which the normal ferrous class is converted to the ferric land. Methaemoglobinaemia causes a left shift in the bend as methaemoglobin does not unload oxygen from haemoglobin.
The other oxygen transport molecules
At that place are ii other oxygen transport molecules that are required knowledge and unremarkably asked about in medical exams, fetal haemoglobin and myoglobin:
Fetal haemoglobin
Fetal haemoglobin (HbF) is the main oxygen transport protein in the man fetus during the last 7 months of development. Information technology persists in the newborn until roughly 6 months of age. HbF has different globin chains to developed haemoglobin (Hb). Whereas adult haemoglobin is composed of ii blastoff and ii beta subunits, fetal haemoglobin is composed of two blastoff and two gamma subunits. This change in the globin concatenation results in a greater affinity for oxygen and allows the fetus to extract oxygen from the maternal circulation. This increased affinity for oxygen means that the oxygen dissociation bend for fetal haemoglobin is shifted to the left of that of adult haemoglobin.
Myoglobin :
The curve for myoglobin lies even further to the left than that of fetal haemoglobin and has a hyperbolic, not sigmoidal, shape. Myoglobin has a very loftier affinity for oxygen and acts as an oxygen storage molecule. It only releases oxygen when the partial pressure of oxygen has fallen considerably. The function of myoglobin is to provide additional oxygen to muscles during periods of anaerobic respiration.
Header image used on licence from Shutterstock
Give thanks you lot to the joint editorial team of www.mrcemexamprep.net for this commodity.

Source: https://www.medicalexamprep.co.uk/understanding-oxygen-dissociation-curve/
0 Response to "what causes oxygen to be released in the tissues? explain your answer."
Post a Comment